Cleanrooms are controlled environments with low levels of pollutants that are used in industries where contamination control is critical. There are different cleanroom classes or standards that specify maximum concentration limits for particles, airborne microbes, surface particles, air flow patterns and other parameters. The cleanroom class depends on the intended use of the cleanroom and the cleanliness requirements of the processes or products inside.
What are the different classes of cleanrooms?
There are several classification systems for cleanrooms. The most commonly used is the ISO classification system which defines the following cleanroom classes:
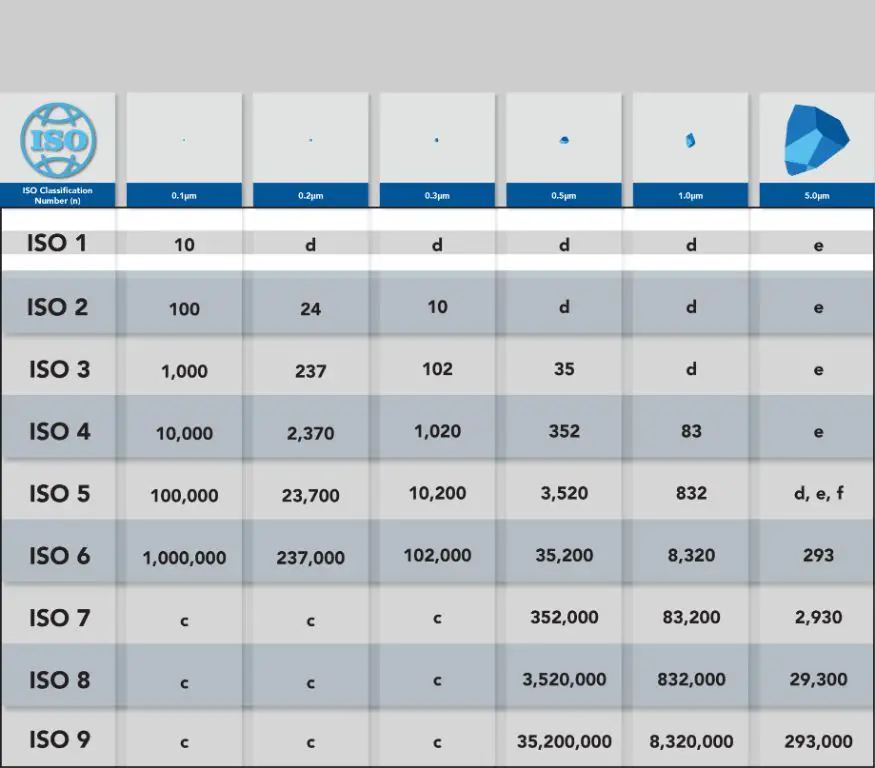
- ISO Class 1: Very high air cleanliness, used for aseptic manufacturing processes. Max allowable particle concentration is 10 particles/m3 for particles ≥0.1 μm.
- ISO Class 2: Used for aseptic filling, biotechnology and pharmaceutical processes. Max particle concentration is 100 particles/m3 for particles ≥0.1 μm.
- ISO Class 3: Cleanrooms for pharmaceutical processes and medical device assembly. Max particle concentration is 1000 particles/m3 for particles ≥0.1 μm.
- ISO Class 4: Used for microelectronics, medical device assembly and preparation of sterile products. Max particle concentration is 10,000 particles/m3 for particles ≥0.1 μm.
- ISO Class 5: Cleanrooms for microelectronics, optical and aerospace industries. Max particle concentration is 100,000 particles/m3 for particles ≥0.1 μm.
- ISO Class 6: Used for assembly and testing of mechanical devices with high cleanliness requirements. Max particle concentration is 1,000,000 particles/m3 for particles ≥0.1 μm.
- ISO Class 7: Used for medical device manufacturing and microelectronics assembly. Max particle concentration is 352,000 particles/m3 for particles ≥0.5 μm.
- ISO Class 8: Suitable for pharmaceutical packaging and medical device assembly. Max particle concentration is 3,520,000 particles/m3 for particles ≥0.5 μm.
- ISO Class 9: Used for precision mechanical device assembly. Max particle concentration is 35,200,000 particles/m3 for particles ≥0.5 μm.
How are cleanroom classes defined and controlled?
Cleanroom classes are defined based on the maximum concentration limits of airborne particles per cubic meter. The smaller the particle size tested for, the higher the cleanroom classification. For instance, ISO Class 5 cleanrooms have a max concentration of 100,000 particles/m3 for particles ≥0.1 μm whereas ISO Class 8 cleanrooms have a limit of 3,520,000 particles/m3 for particles ≥0.5 μm.
To achieve and maintain the required cleanroom class, the following controls are implemented:
- Air filtration using HEPA or ULPA filters to remove particles
- Airflow patterns and air changes per hour to dilute and remove contaminants
- Temperature and humidity control
- Use of specialized cleaning agents and procedures
- Adoption of gowning requirements and entrance protocols
- Monitoring of airborne particle counts and microbiological concentrations
- Proper facility design, construction materials and layout
What are the ISO 14644 Cleanroom Standards?
The ISO 14644 cleanroom standards provide specifications for testing, monitoring and maintenance of cleanrooms to comply with the required ISO classification.
Key aspects covered in ISO 14644 include:
- Test methods to measure air cleanliness by particle concentration, like using optical particle counters.
- Procedures for certification and routine testing of cleanrooms to verify compliance.
- Installation and commissioning requirements for cleanroom equipment like filters and air handling systems.
- Recommended monitoring frequencies for particle counts, airflow velocities, pressure differentials, etc.
- Guidelines for operations like cleaning, gowning, materials storage, etc. to maintain cleanliness.
- Methods for determining air change rates, filter installation leakage and other cleanroom performance parameters.
Adherence to ISO 14644 ensures that the required cleanroom class is consistently achieved to meet process or product cleanliness requirements. The standards cover testing, certification, operation and maintenance procedures in detail.
How do federal standards classify cleanrooms?
US Federal Standard 209E is another cleanroom classification system that was historically used but has now been replaced by the ISO 14644 standards.
It defined airborne particle concentration limits for cleanrooms as follows:
| Class | Max particles/ft3 ≥0.5 μm |
|---|---|
| M1 | 1 |
| M2 | 10 |
| M3 | 100 |
| M4 | 1000 |
| M5 | 10,000 |
| M6 | 100,000 |
Federal Standard 209E classes correspond approximately to the following ISO classifications:
| Federal Standard 209E | Equivalent ISO 14644-1 |
|---|---|
| Class M3.5 | ISO Class 5 |
| Class M4.5 | ISO Class 6 |
| Class M5.5 | ISO Class 7 |
| Class M6.5 | ISO Class 8 |
The federal standard is now obsolete. ISO classifications are more detailed and cover wider particle size ranges, airborne concentrations, operations, maintenance and testing procedures.
What cleanroom classification is used for manufacturing semiconductor chips?
Semiconductor manufacturing involves multiple processes like photolithography, etching, doping, thin film deposition, CMP, die preparation etc. Many of these require very high cleanliness to avoid defects.
Typically, ISO Class 1 cleanrooms are used for critical processes like photolithography, epitaxial silicon growth and mask inspection where sub-micron particle control is needed.
ISO Class 2 cleanrooms are common for wafer processing steps like diffusion, metallization, grind/polish and testing where particulate and molecular contamination must be minimized.
For backend processes like assembly, packaging and testing, ISO Class 6 to ISO Class 8 cleanrooms are often adequate.
So in summary, advanced semiconductor fabs require ISO Class 1 to ISO Class 3 cleanrooms for most fabrication processes, while less stringent ISO Class 5 and above are used for backend stages. Airborne particle counts are continuously monitored to ensure very low defect rates.
What type of cleanroom is required for manufacturing pharmaceutical drug products?
Sterile pharmaceutical products like injectable drugs need to be produced in very high grade cleanrooms to prevent microbial or particulate contamination.
Typical cleanroom classifications for pharma manufacturing include:
- ISO Class 5: For non-sterile steps like bulk powder dispensing/milling.
- ISO Class 7: For product contact steps like capsule filling.
- ISO Class 8: For steps involving exposed product like tablet compression, or equipment sterilization zones.
- ISO Class 7 or 8 with Grade A/B enclosures: For aseptic processing and filling steps. The background room is ISO 7 or 8 while the local enclosure around the exposed product meets ISO 5 (Grade A) particulate standards.
Some critical applications like aseptically filled injectable drugs may require:
- ISO Class 5 for non-product contact areas.
- ISO Class 5 Grade A at filling points.
- ISO Class 5 Grade B in background during filling.
- ISO Class 7 packaging line.
Cleanroom garment standards, disinfection procedures, air exchanges and environmental monitoring are also stringent for pharma cleanrooms to minimize risks of microbial contamination.
What cleanroom class is required for manufacturing medical devices?
Medical device manufacturing covers a wide range of products from simple masks and gowns to complex implantable devices like pacemakers. The cleanroom classification varies based on the device functionality, patient risk factors and regulatory requirements. Some examples are:
- ISO Class 8: For low risk devices like masks, gowns, surgical drapes, etc.
- ISO Class 7: For higher risk devices that contact sensitive tissues. E.g. Intravenous catheters, endotracheal tubes.
- ISO Class 6: For more critical devices like orthopedic implants, cardiac stents, surgical power tools.
- ISO Class 5 with Grade A/B enclosures: Very critical products like implantable electronic devices that go into heart, spine, brain. Grade A at filling/sealing step.
FDA provides guidelines for medical device cleanrooms based on the device’s biological safety category. Particle counts, microbial levels, air exchanges, room pressure differentials and other parameters must comply with recommended standards appropriate to the device risk and category. Cleanliness verification and vigilance is essential for medical device quality assurance.
When are lower grade Cleanrooms Class 100,000 or Class 1,000,000 used?
Relatively ‘dirty’ cleanrooms like ISO Class 5 or Class 100,000 (FS-209E Class M5.5) and ISO Class 6 or Class 1,000,000 (FS-209E Class M6.5) are suitable for applications where high product cleanliness is not required.
Some examples of processes carried out in these lower cleanliness grades are:
- Automotive manufacturing: Engine assembly, transmission assembly, brake systems assembly.
- Aircraft manufacturing: Airframe structure assembly, wing assembly, landing gear installation.
- Spacecraft manufacturing: Structural assembly, rocket engine component fabrication.
- Consumer electronics: TV and home appliance assembly and testing.
- Optics: Assembly and alignment of optical systems and telescopes.
- Mechanical hardware: Gears, ball bearings, valves, pumps assembly and packaging.
- Medical disposables: Surgical gowns and drapes, masks, etc.
Here product or process contamination risks are relatively lower so higher particle levels are permitted. Only basic contamination control is needed, so lower air filtration, simpler garbing and cleaning protocols can be implemented, reducing costs.
When are higher grade Cleanrooms Class 10, 100 or 1000 used?
Ultra-clean environments like ISO Class 4 (Class 10 or FS-209E Class M3.5), ISO Class 5 (Class 100 or FS-209E Class M4.5) or ISO Class 6 (Class 1000 or FS-209E Class M5.5) are necessary for the following:
- Pharmaceutical manufacturing: Sterile filling, powder handling, tablet compression, packaging.
- Medical devices: Implantables, catheters, surgical tools.
- Microelectronics: Wafer fabrication, lithography, diffusion, etching, packaging.
- Optics: Precision cleaning, coating, lithography and assembly of optics.
- Aerospace: Precision cleaning and coating of mirrors, sensors, lasers.
- Laboratory research: Sample preparation, handling and testing; clean culture inoculations.
Here the product or process is very sensitive to particle contamination. Highly controlled environments are needed to minimize risks of defects, failures or loss of sterility assurance.
Conclusion
Cleanroom classification is based on the maximum concentration of airborne particles permitted as per ISO 14644 or the now-obsolete US FS-209E standards. Higher numerical classes indicate cleaner environments with lower particle levels. Cleanliness class must be appropriate for the product or process, balancing contamination risks with operational costs. ISO classes 1 to 3 are used for critical processes in semiconductor, biopharma and medical device industries handling vulnerable materials. ISO classes 6 to 9 are adequate for manufacturing less sensitive mechanical and consumer products. Careful cleanroom design, monitoring and control is vital for compliance with air purity standards.