ISO 14644-2 provides guidance on how to evaluate, monitor and maintain cleanrooms and associated controlled environments. It is part of the ISO 14644 series of standards related to cleanrooms and contaminant control. Here are some key points about ISO 14644-2:
What is the purpose of ISO 14644-2?
The main purposes of ISO 14644-2 are to:
- Specify appropriate cleanliness classifications for cleanrooms and clean air devices
- Establish methods for evaluating and monitoring cleanrooms and clean air devices against these classifications
- Provide guidance on maintenance and related aspects to ensure continued compliance with the specified classifications
By providing a standardized basis for cleanroom classification, evaluation and monitoring, ISO 14644-2 aims to support quality and productivity in sectors where contamination control is important. This includes industries such as pharmaceuticals, medical devices, microelectronics, optics and aerospace.
What are the cleanroom classifications specified in ISO 14644-2?
ISO 14644-2 specifies classifications for air cleanliness in cleanrooms and clean air devices. There are four primary classification levels:
- ISO Class 1: Highest level of air cleanliness. Particulate concentration limit is 10 particles/m3 for particles ≥0.1 μm.
- ISO Class 2: Particulate concentration limit is 100 particles/m3 for particles ≥0.1 μm.
- ISO Class 3: Particulate concentration limit is 1,000 particles/m3 for particles ≥0.1 μm.
- ISO Class 4 to ISO Class 9: Have progressively higher particulate concentration limits, from 10,000 to 1,000,000 particles/m3 for particles ≥0.1 μm.
There are also classifications (ISO Class 5 to ISO Class 9) for larger particle sizes from ≥0.2 μm to ≥5.0 μm. The numeric value indicates the maximum permitted particle concentration for the associated particle size range.
How are cleanrooms evaluated against these classifications?
ISO 14644-2 outlines several techniques for evaluating the cleanliness classification of a cleanroom or clean air device, including:
- Particle counting: Using an optical particle counter to measure airborne particle concentrations inside the cleanroom or device. Results are checked against limit values for the target classification.
- Airflow visualization: Using smoke trails to observe airflow patterns. This can identify potential causes of turbulence or stagnation that may increase particle counts.
- Airflow velocity measurements: Using anemometers to map velocities across the cleanroom to ensure maintenance of desired air movement. Lower velocities can allow particle buildup.
- Containment leak testing: Using particle counters or chemical detectors to check for leaks in the cleanroom enclosure under positive or negative pressure.
What is involved in routine cleanroom monitoring?
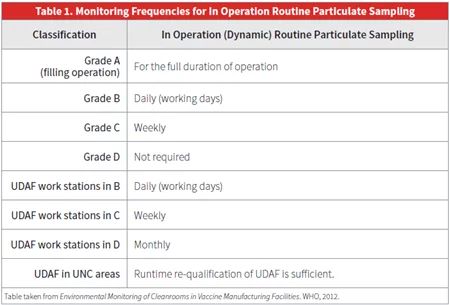
To maintain continued compliance with specified classifications, ISO 14644-2 requires routine monitoring of parameters such as:
- Airborne particle concentrations
- Airflow velocities
- Differential pressures between clean zones
- Temperature and humidity
- Air change rates
- Filter installation conditions
Monitoring frequencies range from continuous for particulate counts, to daily, weekly or less often for other parameters. Results should be documented and checked against acceptance criteria. Monitoring methods must meet performance requirements outlined in ISO 14644-2.
How can cleanrooms be maintained to preserve classifications?
ISO 14644-2 provides guidance on maintaining facilities to sustain target cleanliness classifications. Recommended maintenance activities include:
- Regular filter servicing and replacement regimes
- Visual inspection of facilities for integrity breaches
- Cleaning procedures and schedules for different surfaces
- Preventative maintenance on air handling units and other equipment
- Testing and certification of utilities like pure water
- Staff training on contamination control disciplines
- Calibration and servicing of monitors and instruments
The standard states that any maintenance intersecting the cleanroom should be performed using contamination control measures outlined in ISO 14644-4. Proper maintenance is essential for cleanliness classification compliance.
What are some key requirements before classification testing?
ISO 14644-2 sets out requirements to be fulfilled prior to formal cleanroom classification testing. These include:
- Cleanroom construction and commissioning is complete
- Air handling and filtration systems are operating stably
- Routine monitoring shows continued compliance with specifications
- Cleaning, sanitization, utilities supply etc. are in place
- Planned operations, furniture, equipment are installed
- Monitoring equipment calibrated and located appropriately
- Sufficient air volume exchanges achieved for target classification
Adhering to these prerequisites helps ensure the cleanroom is ready for formal classification testing.
What are some limitations of ISO 14644-2 classifications?
While ISO 14644-2 provides a useful standard, some limitations exist:
- Classifications only consider airborne particles – does not account for chemical or biological contamination.
- Limited to sub-micron particle sizes – larger particles may also be detrimental.
- Steady-state conditions – does not evaluate disturbances like human interventions.
- Not a process specification – does not guarantee product quality requirements are met.
- Limited sampling – particle counts reflect small sample volumes.
Due to these factors, organizations should view ISO 14644-2 classification as one input to quality management rather than the sole determinant of cleanliness.
What are some key terms used in ISO 14644-2?
Some important definitions of terms used in the standard include:
- Cleanroom: Room with controlled environment for minimizing introduction, generation and retention of particles.
- Clean air device: Enclosure that provides local ultra-clean air environment for contamination control.
- Primary engineering control systems: HVAC and filtration systems used to achieve air cleanliness classifications.
- At-rest state: Cleanroom or clean air device operating in normal production mode without human interventions.
- As-built state: Condition after construction but before production equipment is installed.
- As-operated state: Condition when facility is fully equipped and operating in production mode.
What is the scope of ISO 14644-2?
ISO 14644-2 specifically covers:
- Classification of air cleanliness for cleanrooms and clean air devices
- Test methods to demonstrate compliance with these classifications
- Guidance for evaluating and monitoring facilities against classifications
- Maintaining facilities in qualified state
- Key requirements prior to formal classification testing
It does not cover aspects such as:
- Cleanroom design and construction
- Selection of air filtration systems
- Contamination control of utilities like pure water
- Setting contamination limits for specific processes
- Cleanroom clothing and human behaviors
These are addressed through other standards in the ISO 14644 series.
How was ISO 14644-2 developed?
ISO 14644-2 was developed by the International Organization for Standardization (ISO) Technical Committee (TC) 209 Working Group (WG) 2. Key milestones in its development include:
- 1999 – Original version published
- 2000 – Technical Corrigendum 1 issued
- 2005 – Minor revisions incorporated in Edition 2
- 2015 – Current Edition 3 published
The standard continues to be reviewed and updated periodically to reflect evolving technologies and industry needs related to cleanroom air cleanliness classification and control.
How does ISO 14644-2 relate to other cleanroom standards?
ISO 14644-2 is part of the ISO 14644 series of cleanroom standards, which is divided into four primary parts:
- ISO 14644-1: Classification of air cleanliness and related cleanroom classes
- ISO 14644-2: Specifications for monitoring and periodic testing to prove continued compliance
- ISO 14644-3: Metrology and test methods
- ISO 14644-4: Design, construction, start-up, and qualification of facilities
ISO 14644-2 works in conjunction with these sibling documents for a comprehensive approach to cleanroom management. There are also separate ISO standards governing cleanroom operations, garments, sterile products manufacturing, etc.
How does ISO 14644-2 align with US and European standards?
ISO 14644-2 is generally aligned with related cleanroom standards from the US and EU, including:
- US Federal Standard 209E – Airborne particulate cleanliness classes
- EN ISO 14644-1 – Classification of airborne particulates
- EN ISO 14644-2 – Specifications for testing and monitoring to prove continued compliance
- IEST-RP-CC012 – Considerations for cleanroom design
While minor differences exist, these standards establish similar classification levels and share the goal of enabling cleanroom control to meet contamination requirements.
Is certification to ISO 14644-2 required?
Certification to ISO 14644-2 is not required by the standard itself. However, organizations may choose to seek third-party ISO 14644-2 certification from accredited auditing bodies to demonstrate compliance to customers or regulatory authorities.
For highly regulated industries like pharmaceutical manufacturing, certification can be an important credential to prove a quality system is in place for contamination control. However, certification involves added cost and ISO 14644-2 conformance can also be shown through careful monitoring and documentation.
How are cleanrooms qualified and re-qualified to ISO 14644-2?
Initial cleanroom qualification per ISO 14644-2 involves:
- Verifying all specifications are met for construction, airflow, filtration, utilities, monitoring, etc.
- Conducting formal particle counting tests in the at-rest state
- Documenting the maximum calculated concentration for the target classification
For re-qualification after major changes, this full verification and testing process is repeated. For routine re-qualification (e.g. annually), monitoring data demonstrating continuous compliance can suffice along with visual inspection and potentially sample particle counting tests.
What are some common pitfalls when applying ISO 14644-2?
Some problems that can arise in application of ISO 14644-2 include:
- Assuming classification alone ensures product quality
- Not performing regular monitoring or maintenance after initial qualification
- Improper use of monitoring methods and instruments
- Inadequate sample sizes or sampling durations during counting tests
- Failing to investigate or mitigate increasing particle trends
- Modifying facilities without re-qualification considerations
Proper personnel training, documentation, instrument calibration, and communication between departments is key to avoiding these issues.
What are some recent trends related to ISO 14644-2?
Some current trends around ISO 14644-2 include:
- Increased automation of cleanroom monitoring to provide 24/7 data
- More interest in isolator technology for segregating human operators
- Use of risk-based qualification methods beyond ISO 14644-2 requirements
- Shift from particulate-only focus to include chemical and biological contamination
- Addition of complete cleanroom certification services by testing companies
- Growing use in food, electronics, and aerospace sectors beyond pharmaceuticals
The principles of ISO 14644-2 remain relevant while techniques continue advancing to meet industry challenges.
Conclusion
ISO 14644-2 provides standardized methods for classifying, evaluating, monitoring and maintaining cleanrooms and clean air devices. By establishing acceptance criteria and measurement procedures, it promotes quality and productivity for industries where contamination control is imperative. While certification is optional, adherence to ISO 14644-2 demonstrates commitment to best practices in cleanroom management.
With environmental control increasingly critical for advanced manufacturing, healthcare, and other fields, the principles and methods of ISO 14644-2 will continue providing an important benchmark for cleanliness long into the future.